21 Children Dead from Toxic Cough Syrup in Madhya Pradesh
The Live Info Media
At least 21 children in Madhya Pradesh’s Chhindwara district and nearby areas died from acute kidney failure after consuming Coldrif syrup contaminated with diethylene glycol, manufactured by Sreesan Pharma in Tamil Nadu. The state banned the syrup on October 5, and the central health ministry advised against prescribing cough medications to children under two, following tests revealing 364 violations at the facility. Five more children are in critical condition as investigations continue, highlighting ongoing issues in pharmaceutical regulation after similar incidents in 2022.
A deepening mystery surrounds the cause of death for twelve children across Madhya Pradesh and Rajasthan, with initial suspicions of contaminated cough syrup now being questioned by new laboratory findings. The death toll doubled to twelve on Friday, with nine fatalities in Madhya Pradesh’s Chhindwara district. While early biopsy reports from Chhindwara had suggested Diethylene Glycol (DEG) contamination in the cough syrup given to the children, the Ministry of Health and Family Welfare announced that their laboratory tests on collected syrup samples detected neither DEG nor Ethylene Glycol (EG).
With the suspected cause now uncertain, a multi-disciplinary team of experts from various national health and research institutions is urgently investigating alternative possibilities, including contaminated water, disease vectors, and respiratory pathogens. Adding a new layer to the mystery, the National Institute of Virology (NIV) in Pune detected Leptospirosis, a type of bacterial infection, in the blood sample of one affected child. Samples of water and various biological specimens are under further investigation to pinpoint the root cause of the children’s acute kidney injuries and deaths.
In response to the tragedy, the Directorate General of Health Services issued a public health advisory cautioning against the common practice of giving children cough syrups meant for adults. The advisory stressed that most cough illnesses in children resolve naturally without medication and strongly recommended that cough and cold medicines should not be given to children under two years old. This guidance aims to prevent potential harm from inappropriate drug use while the main investigation continues.
Testing of various medicines is ongoing. The Madhya Pradesh drug controller stated that initial results for antibiotic and paracetamol samples came back clean. However, the results for two specific cough syrup brands, Coldrif and Nexa/NEXTRO DS, are still pending. These two syrups, manufactured by companies based in Tamil Nadu and Himachal Pradesh, respectively, were consumed by the child victims and are a key focus of the detailed inquiry being conducted by state and central drug authorities.
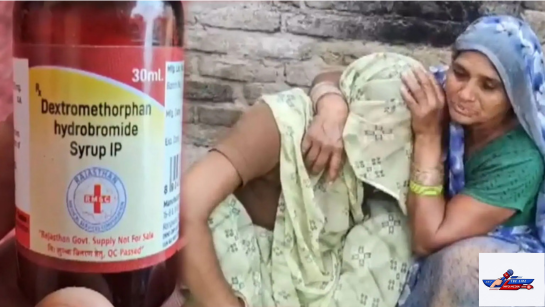
In Rajasthan, where three children have died, the health ministry noted that a Dextromethorphan-based syrup linked to two cases did not contain common DEG/EG sources. However, they pointed out that this type of formulation is not recommended for pediatric use, suggesting unsupervised or inappropriate use may be a factor. With 13 children still hospitalized in Nagpur, four in serious condition, authorities are racing to identify the cause, screen affected children, and urge parents to avoid unprescribed treatment to prevent further fatalities.







